Impaired theta phase coupling underlies frontotemporal dysconnectivity in schizophrenia
Rick A. Adams,1,2,3,4,5,* Daniel Bush,1,6,* Fanfan Zheng,1,7,* Sofie S. Meyer,1,6 Raphael Kaplan,5,8 Stelios Orfanos,9,10 Tiago Reis Marques,11,12 Oliver D. Howes 11,12 and Neil Burgess 1,5,6
*These authors contributed equally to this work.
1 Institute of Cognitive Neuroscience, University College London, 17 Queen Square, London, WC1N 3AZ, UK
2 Division of Psychiatry, University College London, 149 Tottenham Court Road, London, W1T 7NF, UK
3 Max Planck-UCL Centre for Computational Psychiatry and Ageing Research, 10-12 Russell Square, London, WC1B 5EH, UK
4 Centre for Medical Image Computing, Department of Computer Science, University College London, Malet Place, London, WC1E7JE, UK
5 Wellcome Centre for Human Neuroimaging, University College London, 12 Queen Square, London, WC1N 3BG, UK
6 Queen Square Institute of Neurology, University College London, London, WC1N 3BG, UK
7 Brainnetome Center, Institute of Automation, Chinese Academy of Sciences, 95 Zhongguancun East Road, 100190 Beijing, China
8 Kavli Institute for Systems Neuroscience, Norwegian University of Science and Technology, Trondheim, Norway
9 South West London and St George’s Mental Health NHS Trust, Springfield University Hospital, 61 Glenburnie Rd, London SW177DJ, UK
10 Department of Forensic and Neurodevelopmental Sciences, Institute of Psychiatry, Psychology, and Neuroscience, King’s College London, De Crespigny Park, Denmark Hill, London SE5 8AF, UK
11 Institute of Clinical Sciences, Faculty of Medicine, Imperial College London, Hammersmith Hospital, London, W12 0NN, UK
12 Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience (IoPPN), King’s College London, London, SE5 8AF, UK
Abstract
Frontotemporal dysconnectivity is a key pathology in schizophrenia. The specific nature of this dysconnectivity is unknown, but animal models imply dysfunctional theta phase coupling between hippocampus and medial prefrontal cortex (mPFC). We tested this hypothesis by examining neural dynamics in 18 participants with a schizophrenia diagnosis, both medicated and unmedicated; and 26 age, sex and IQ matched control subjects. All participants completed two tasks known to elicit hippocampal-prefrontal theta coupling: a spatial memory task (during magnetoencephalography) and a memory integration task. In addition, an overlapping group of 33 schizophrenia and 29 control subjects underwent PET to measure the availability of GABAARs expressing the a5 subunit (concentrated on hippocampal somatostatin interneurons). We demonstrate—in the spatial memory task, during memory recall—that theta power increases in left medial temporal lobe (mTL) are impaired in schizophrenia, as is theta phase coupling between mPFC and mTL. Importantly, the latter cannot be explained by theta power changes, head movement, antipsychotics, cannabis use, or IQ, and is not found in other frequency bands. Moreover, mPFC-mTL theta coupling correlated strongly with performance in controls, but not in subjects with schizophrenia, who were mildly impaired at the spatial memory task and no better than chance on the memory integration task. Finally, mTL regions showing reduced phase coupling in schizophrenia magnetoencephalography participants overlapped substantially with areas of diminished a5-GABAAR availability in the wider schizophrenia PET sample. These results indicate that mPFC-mTL dysconnectivity in schizophrenia is due to a loss of theta phase coupling, and imply a5-GABAARs (and the cells that express them) have a role in this process.
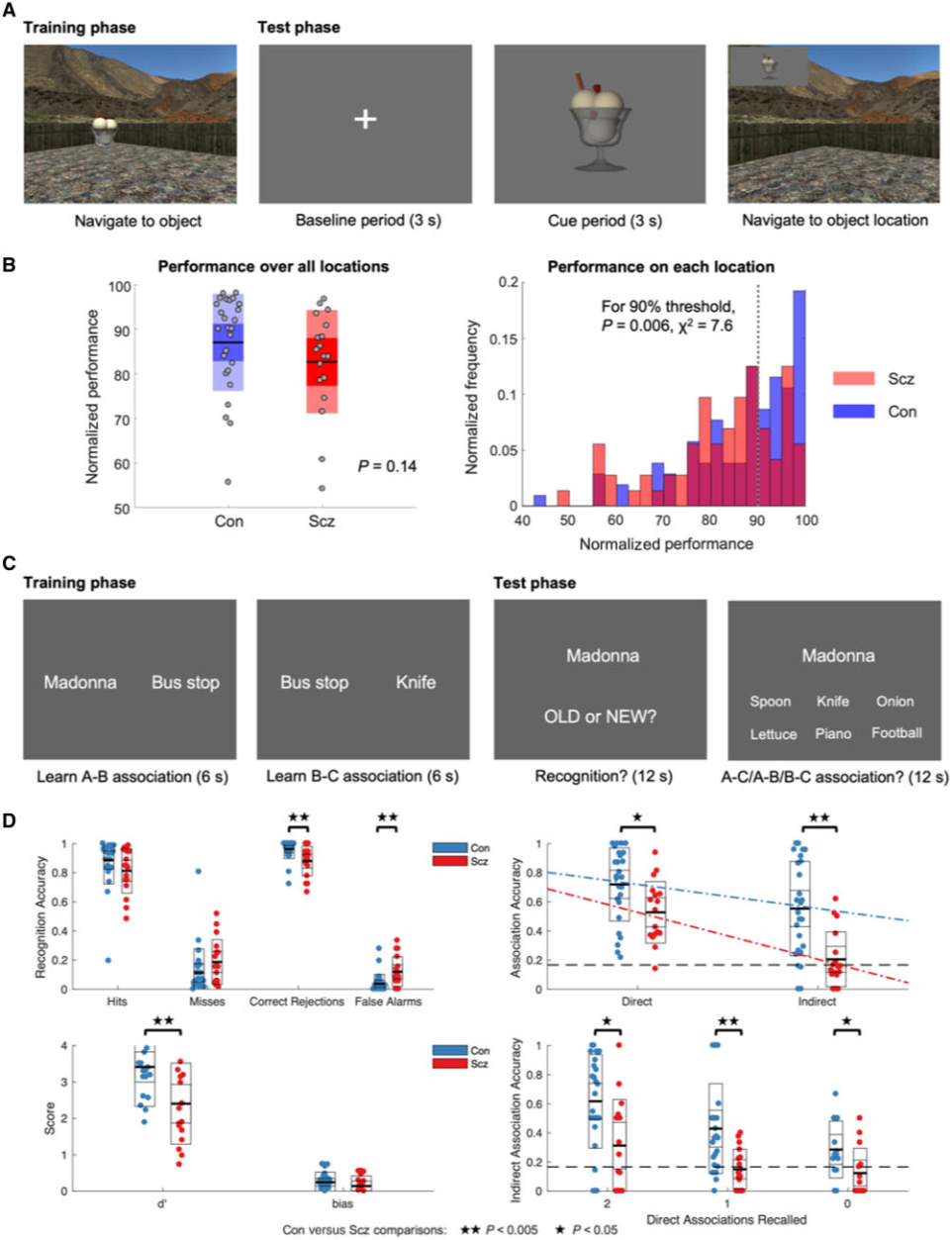
Figure 1. Task structure and behavioural findings.
