FRUIT Protocol
Fructose and urea induced transparency- FRUIT Protocol
If you have any question, please contact to Dr. Bing Hou
Please cite the following paper if you use it in your research.
Hou, B., Zhang, D., Zhao, S., Wei, M., Yang, Z., Wang, S., Wang, J., Zhang, X., Liu, B., Fan, L., Li, Y., Qiu, Z., Zhang, C., Jiang, T. Scalable and DiI-compatible optical clearance of the mammalian brain. Front Neuroanat, 9:19, 2015.
Declaration: This protocol can be used freely for academic, non-profit purposes.
If you intend to use it for commercial development, please, contact us.
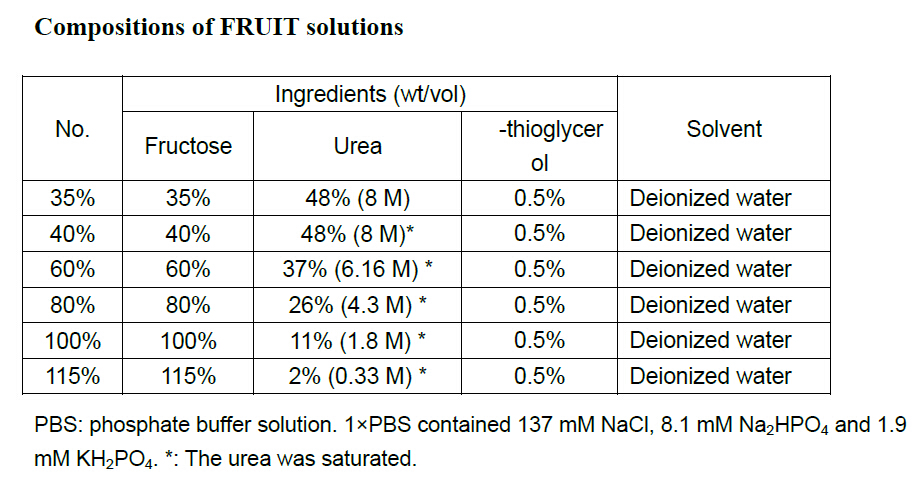
Compositions of FRUIT solutions
Optimal FRUIT procedure for the mouse brain
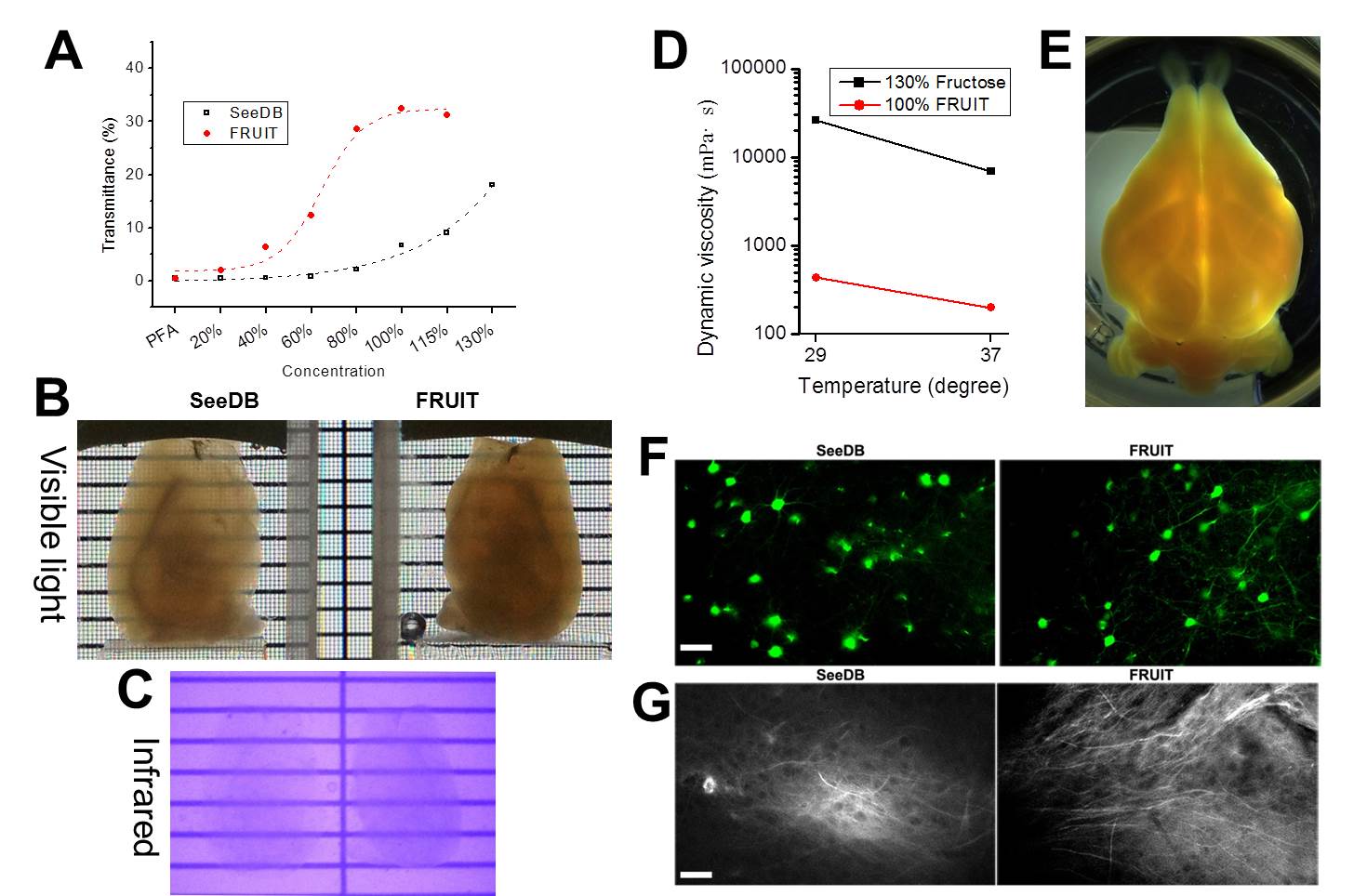
Adult mice over 70 days of age were deeply anesthetized with an intraperitoneal overdose of sodium pentobarbital (70 mg/kg body weight) and transcardially perfused with 1×PBS followed by 4% (wt/vol) paraformaldehyde (PFA) in 1×PBS. The whole brains were excised and then post-fixed in the same fixative at 4°C overnight. The brain samples were serially incubated in 20-30 ml of 35%, 40% and 60% (wt/vol) FRUIT, each for 8 h in 50-ml conical tubes with gentle rotation (~4 rpm) at 37°C. The samples were then incubated in 80% (wt/vol) FRUIT for 12 h and finally 100% FRUIT for 24 h with gentle rotation at 37°C. In the case of the Thy1-YFP (line H) mice, the samples were covered with foil and protected from light during clearing. Incubation at a temperature over 37°C is not recommended due to concern regarding the breakdown of FRUIT. Samples could be stored in 100% (wt/vol) FRUIT over 2 months at 4°C.
It should be noted that the optimal procedure might vary depending on animal age, tissue volume, fixation time, solution temperature and other factors. For example, the reduced starting concentration was recommended for young mouse brains.
Perfusion-assisted clearing using FRUIT
For perfusion-assisted clearance of the brain, 3 month old adult rabbits were anesthetized with an intravenous injection of sodium pentobarbital (35 mg/kg body weight) through the auricular vein. The rabbit was secured in a supine position and the common carotid arteries and internal jugular veins were exposed. The bilateral common carotid arteries were ligated and the internal jugular veins were opened. A needle was inserted into each common carotid artery at the distal segment to allow for cerebral perfusion of 1×PBS followed by 4% (wt/vol) PFA in 1×PBS. The rabbit brain was then sequentially perfused with 50 ml of 20%, 40%, 60%, 80% and 100% (wt/vol) FRUIT solutions through an injection pump (ZT-500A1, Z&T Medical Treatment, Shenzhen, China) at a flow rate of 5~10 ml/h at room temperature. The tissue transparency would be further improved after the excised rabbit brain was immersed in 100% (wt/vol) FRUIT solution for 24 hours at 37°C.